For NHS Trusts
What are we doing?
Repeated self-harm is common and is associated with poor quality of life and with an increased risk of suicide. There is currently no treatment, deliverable in mainstream mental health services, that works well for individuals with a history of multiple repetition. The aim of this programme of research is to develop and evaluate a new approach for helping individuals who repeatedly self-harm - based upon changing existing therapies so they are more useful in helping people who repeatedly self-harm.
Current treatment tends to focus on the act of self-harm as a symptom of underlying distress. However, personal accounts of reasons for repetition also identify that self-harm has a purpose for the individual, such as helping a person to feel a stronger person or feel more in control of their life. We wish to develop a new approach that can accommodate all potential motivations and allow therapists and service users to identify, and work with, these purposeful functions. A goal of therapy is then to explore what the purposes of self-harm say about an individual’s values and help consider new ways to achieve change that reflects those values.
Following earlier studies identifying reasons for self-harm, and reasons to stop self-harm, we have held several workshops that brought together therapists and people with experience of self-harm, and we have now developed three modified therapies. We will be implementing these therapies in a national randomised controlled trial, to assess their acceptability to patients and therapists and their cost-effectiveness, as well as how deliverable these therapies are within the NHS.
We carried out a feasibility study across 3 sites in 2020 and launched the main clinical trial in 2021.
Who are we looking for and what will be involved?
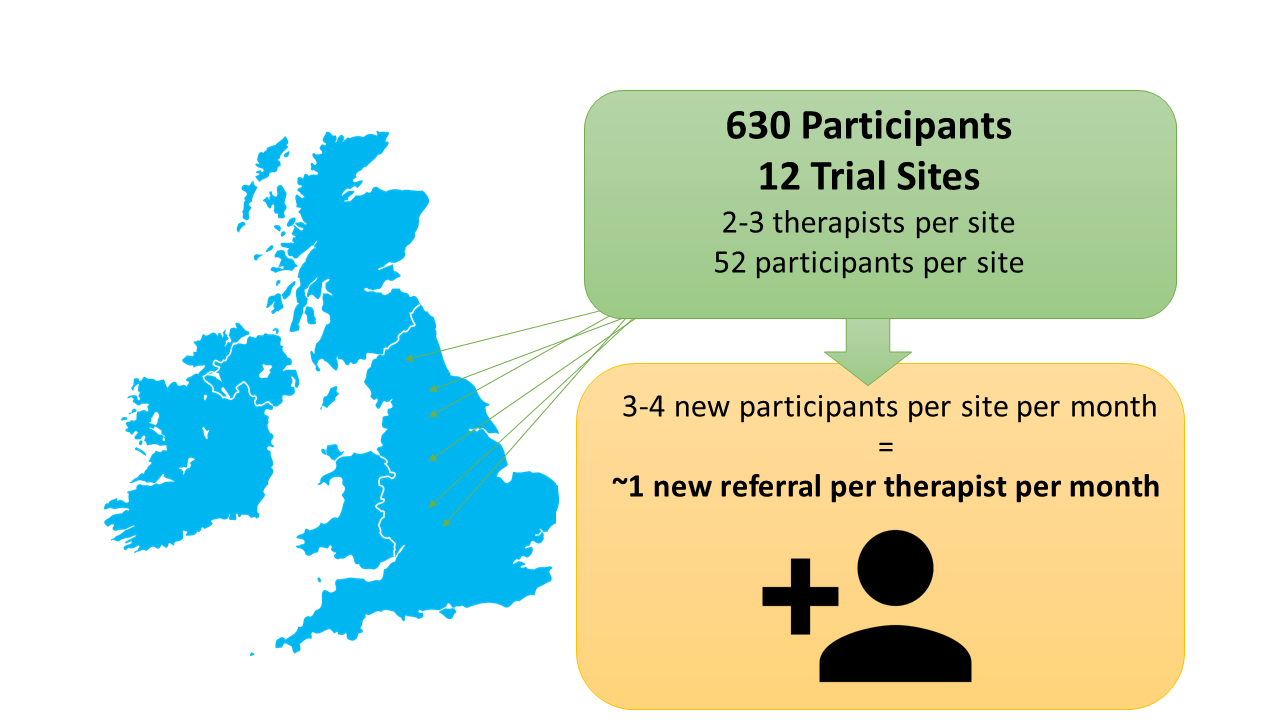
For the main clinical trial, we want to include 12 sites across England, with 2-3 therapists per site. We are looking to work with mental health nurses to act as therapists – we will provide training and supervision in the therapies. We started recruiting to the trial in 2021.
We are aiming to recruit 630 participants over the 12 sites, with approximately 52 participants at each trial site. Each participant will attend 12 sessions over 3-4 months. The trial will be a randomised controlled trial, with half the participants receiving the revised therapy and half receiving NICE-recommended standard care.
We anticipate this to mean than each site will randomise 3-4 participants per month into the trial, which would equate to one new referral per month for each therapist, if there are two intervention therapists per site.
Revised Therapies
Participants will receive one of three therapies modified to include new assessment. Details of the therapies can be found here.
Standard Care
NICE recommends that everybody seen after self-harm receives both a risk assessment and an assessment of needs. It is on the basis of the assessment of need, rather than either risk assessment alone or diagnosis alone, that a care plan is drawn up in collaboration with the person who has self-harmed. We will work with each participating site to identify how these recommendations are implemented and recorded in each site, so that we can monitor this part of the trial as well as the therapy.
See NICE self-harm standard care guidelines for more information.
Why take part?
Training will be provided to all participating therapists and to additional staff who may need to act as back-up in the event of sickness or other absence. This training and the associated supervision will increase the skill set of staff in the Trust. The general therapeutic approach will be readily modifiable to suit other presentations, and so the training will have generalisable benefits for the team.
FReSH START is funded by NIHR Programmes for Applied Research and all participating Trusts receive financial support for participation from NIHR funding streams.
For further information, or expressions of interest, please contact freshstart@leeds.ac.uk
